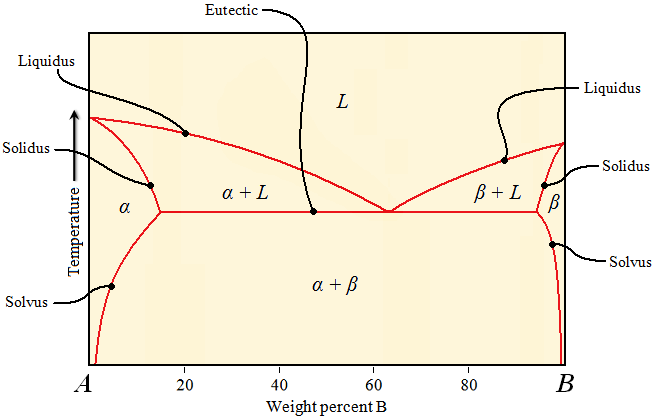
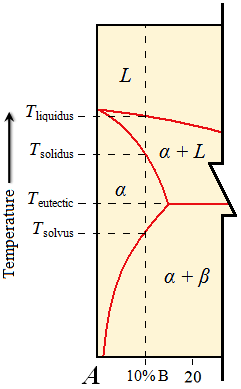
 | | Liquidus, solidus, and solvus temperatures will correspond to a specific composition of interest. In this case, the composition of interest is 10% B. B is the component that is at the other end of the phase diagram that is cut off. Therefore, the liquidus, solidus, and solvus temperatures are those in which their respective lines intersect the vertical dashed line at the composition of interest. The eutectic temperature is the same, regardless of the composition. |
|