For the Dalton model of gas mixtures, the properties of each component of the mixture are considered as though each component exists separately and independently at the temperature and volume of the mixture. It is also assumed that both the gas mixture and the separated components behave according to the ideal gas model. In general, it would be preferable to analyze gas mixture behavior on a mass basis. However, in this particular case it is more convenient to use a mole basis, since the gas constant is then the universal gas constant for each component and also for the mixture. So, for the mixture:
PV = n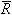 T
T
where:
n = nA + nB
so for the components A and B:
PAV = nA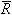 T
T
and
PBV = nB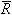 T
T
substituting for the n values:
since all terms cancel besides the pressures:
P = PA + PB
where P is the mixture pressure and PA and PB are referred to as partial pressures. Thus, for a mixture of ideal gases, the pressure is the sum of the partial pressures of the individual components, where,
PA = yAP & PB = yBP
where yA and yB are the mole fractions for each component, so:
Determining Energy, Enthalpy, and Entropy of Ideal Gases
The Dalton model proves useful in the determination of these quantities because the assumption is made that each component behaves as though it occupies the whole volume by itself. Therefore, the internal energy, enthalpy, and entropy may be evaluated as the sum of the respective properties of the constituent gases at the condition at which the components exist in the mixture. Because the internal energy and enthalpy are functions only of temperature for ideal gases, it follows that for a mixture of components A and B, on a mass basis:
U = mu = mAuA + mBuB = m(cAuA + cBuB)
and
H = mh = mAhA + mBhB = m(cAhA + cBhB)
The quantities uA, uB, hA, hB, are the ideal gas properties of the components at the temperature of the mixture. For a process involving a change of temperature, the changes in these values may be evaluated by using three available methods. The above equations could also be expressed as the sums of the componenet mole fractions and properties per mole.
Renaming the gas constant R to be Rmix and from the ideal gas equation:
| Rmix = | | ( | | ) | = | | (n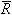 ) = ) = | 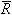 | | Mmix |
|
 T
T T
T T
T T
T
 T
T
 T
T
 ) =
) =