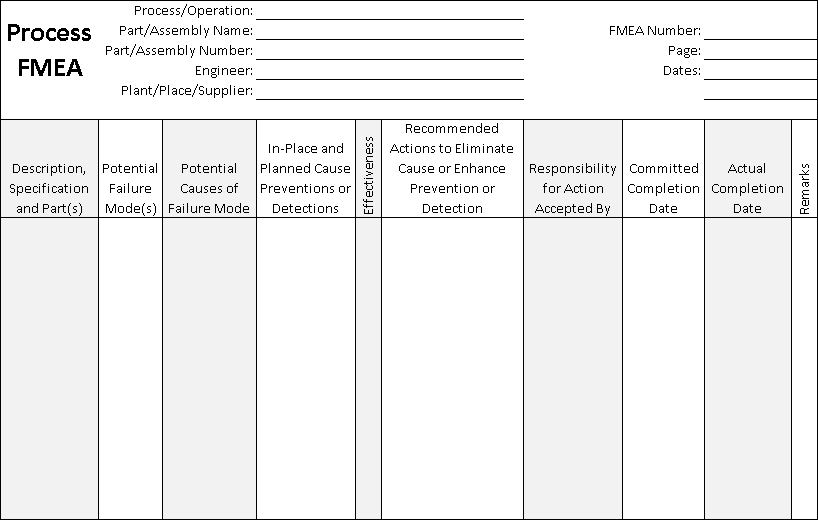
| Process FMEA Form |
| Ranking | Criteria | |
| Verbal | Rate of Cause Occurrence | |
| 1 | The prevention or detection measure is sufficient. The spec will be met consistently. | 1 in ____ |
| 2 and 3 | There's a small chance that the spec will not be met. | 1 in ____ |
| 4, 5, and 6 | Moderate chance. | 1 in ____ |
| 7 and 8 | High chance. | 1 in ____ |
| 9 | Very high chance. | 1 in ____ |
| 10 | The prevention or detection measure is completely ineffective. | 1 in ____ |