The contact angle may also be referred to as the dihedral angle.
The closer a material's contact angle is to zero, the better it's wetting ability. A material has excellent wetting ability if its contact angle is close to zero.
| Wetting | Nonwetting |
 |  |
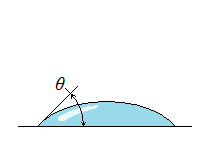 | 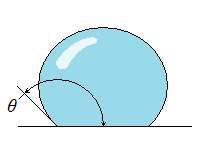 |
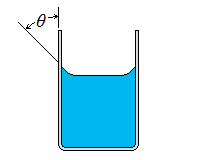
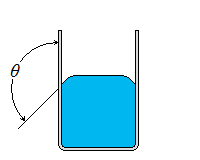
| Wetting, Nonwetting, and Contact Angle |
| It is commonly observed that water in a glass container curves up slightly at the edges where it touches the glass surface; but the opposite occurs for mercury: it curves down at the edges. This effect is usually expressed by saying that water wets the glass (by sticking to it) while mercury does not. The strength of the capillary effect is quantified by the contact (or wetting) angle θ, defined as the angle that the tangent to the liquid surface makes with the solid surface at the point of contact. The surface tension force acts along this tangent line toward the solid surface. A liquid is said to wet the surface when θ < 90° and not to wet the surface when θ > 90°.
The contact angle may also be referred to as the dihedral angle. The closer a material's contact angle is to zero, the better it's wetting ability. A material has excellent wetting ability if its contact angle is close to zero. |
|