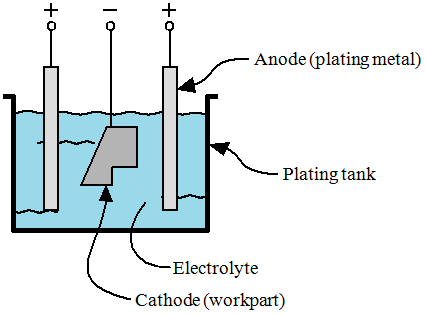
| Electroplating |
| Volume of metal plated | ||||||
| Plating thickness |
| A | surface area of plated part, mm2 (in2) |
| C | plating constant, which depends on electrochemical equivalent and density, mm3/amp-s (in3/amp-min) |
| d | plate thickness, mm(in) |
| E | cathode efficiency |
| I | current, amps |
| t | time during which current is applied, s (min) |
| V | volume of metal plated, mm3 (in3) |
| (Eq1) | V = CIt |
| (Eq2) | V = ECIt |
| (Eq3) |
|
| Coating Designation | Thickness μm (minimum) | Thickness Inch (minimum) |
| Fe/Zn 12 | 12 | 0.0005 |
| Fe/Zn 8 | 8 | 0.0003 |
| Fe/Zn 5 | 5 | 0.0002 |
| Fe/Zn 3 | 3 | 0.0001 |
| Designation | Type | Typical Appearance |
| A | Clear | Transparent colorless with slight iridescence |
| B | Blue-Bright | Transparent with a bluish tinge and slight iridescence |
| C | Yellow | Yellow iridescent |
| D | Opaque | Olive green, shading to brown or bronze |
| E | Black | Black with slight iridescence |
| F | Organic | Any type of the above plus organic topcoat |