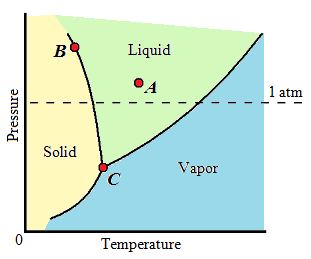
| Gibbs Phase Rule |
| F = C − P + 2 | Gibbs Phase Rule |
| F | number of degrees of freedom, or the amount of variables, such as temperature, pressure, or composition, that are allowed to change independently without changing the number of phases in equilibrium |
| C | number of components, usually elements or compounds, in the system |
| P | number of phases present |