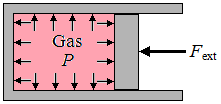
 | | Consider a gas contained in a cylinder fitted with a movable piston, as shown. The pressure exerted by the gas on all its boundaries is the same, assuming that the gas is in an equilibrium state. This pressure is fixed by the external force acting on the piston, since there must be a balance of forces for the piston to remain stationary. Thus, the product of the pressure and the movable piston area must be equal to the external force. If the external force is now changed, in either direction, the gas pressure inside must accordingly adjust, with appropriate movement of the piston, to establish a force balance at a new equilibrium state. As another example, if the gas in cylinder is heated by an outside body, which tends to increase the gas pressure, the piston will move insttead, such that the pressure remains equal to whatever value is required by the external force. |

