Quick
A reversible process is a process that can be reversed by means of infinitesimal changes in some property of the system without loss or dissipation of energy, and can be reversed without causing change in the surroundings.
Details
A reversible process is a process that can be reversed by means of infinitesimal changes in some property of the system without loss or dissipation of energy. Due to these infinitesimal changes, the system is in thermodynamic equilibrium throughout the entire process. In a reversible cycle, the system and its surroundings will be exactly the same after each cycle.
The process in which the system and surroundings can be restored to the initial state from the final state without producing any changes in the thermodynamic properties of the surroundings is called as the reversible process. For the system to undergo reversible change, it should occur infinitely slowly due to an infinitesimal gradient. During a reversible process all the changes in a state that occurrs in a system are in thermodynamic equilibrium with each other. Thus there are two important conditions for the reversible process to occur. First, the process should occur in infinitesimally small time and secondly all the initial and final state of the system should be in equilibrium with each other. In practice the reversible process never occurs, thus it is an ideal or hypothetical process.
Example
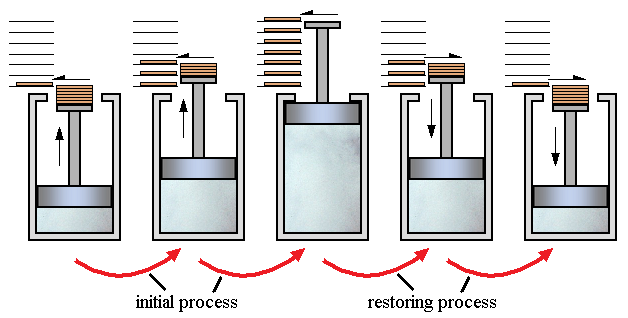
In the following figure, the gas in the cylinder comprises the system, and the piston is loaded with a number of weights. The weights may be slid off horizontally one at a time, allowing the gas to expand and do work in raising the weights that remain on the piston. As the size of the weights is made smaller and their number is increased, a reversible process is approached, for at each level of the piston during the reverse process there is a small weight that is exactly at the level of the platform and thus can be placed on the platform without requiring work. In the limit, therefore, as the weights become very small, the reverse process can be accomplished in such a manner that both the system and surroundings are in exactly the same state they were initially. In theory there is no heat loss or work performed. This may be hard to visualize because how can the weights move without work being performed, but as the weights approach 0 in weight, the work approaches 0. The following schematic represents a process that approaches a reversible processes if the total mass of the weights stays the same, but the mass of the weights themselves decrease, thus meaning the total number of weights also increase. Mathematically, if the mass of an individual weight is almost zero, and the total number of weights is almost infinity, then the process would almost be a reversible process.
⇐ Prev Lesson: Irreversible Process
