| Control Volume Using Mass Continuity and Ideal Gas Law |
Prequisite Knowledge
Ideal Gas Equation
Problem
A hot air heating system (like a furnace) takes in 0.2 m3/s air at 100 kPa and 20°C. The air is heated to 50°C and the flow is delivered at 115 kPa to a duct 0.18 m by 0.24 m. What is the velocity in the duct?
Solution
First, you know you are dealing with air, so be thinking ideal gas law. The heating means the gas expands, the pressure increase means the gas compresses, so the volume flow rate will be different at the inlet and outlet. Second, be thinking mass continuity, because there is an inlet and an outlet. Always, if a system is not accumulating mass, then mass in equals the mass out. The volume flow rate going in will not necessarily equal the volume flow rate going out.
It is helpful to draw a picture with the inlet and outlet conditions.
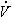 1 = 0.2 m3/s 1 = 0.2 m3/s
P1 = 100 kPa
T1 = 20°C
|

|
A2 = 0.18 m × 0.24 m = 0.0432 m2
P2 = 115 kPa
T2 = 50°C
v2 = ?
|
The temperatures should be converted to Kelvin, so:
T1 = 20°C + 273.15 = 293.15K
and,
T1 = 50°C + 273.15 = 323.15K
The mass flow rate at the inlet can be found using a modified form of the ideal gas equation:
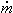 = = | P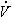 | | RT |
|
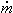 1 = 1 = | | (100 kPa)(0.2 m3/s) | | (0.287 kN m/kg K)(293.15 K) |
| = 0.2377 kg/s |
from mass continuity
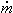 1 =
1 = 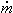 2
2
Because the exit velocity is:
The specific volume needs to be found:
| υ2 = | | (0.287 kN m/kg K)(323.15 K) | | 115 kPa |
| = 0.80647 m3/kg |
Therefore, the velocity is:
| v2 = | | (0.80647 m3/kg)(0.2377 kg/s) | | 0.0432 m2 |
| = 6.36 m/s |
v2 = 4.437 m/s
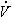 1 = 0.2 m3/s
1 = 0.2 m3/s

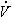 1 = 0.2 m3/s
1 = 0.2 m3/s

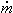 =
=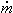 1 =
1 =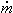 1 =
1 = 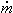 2
2